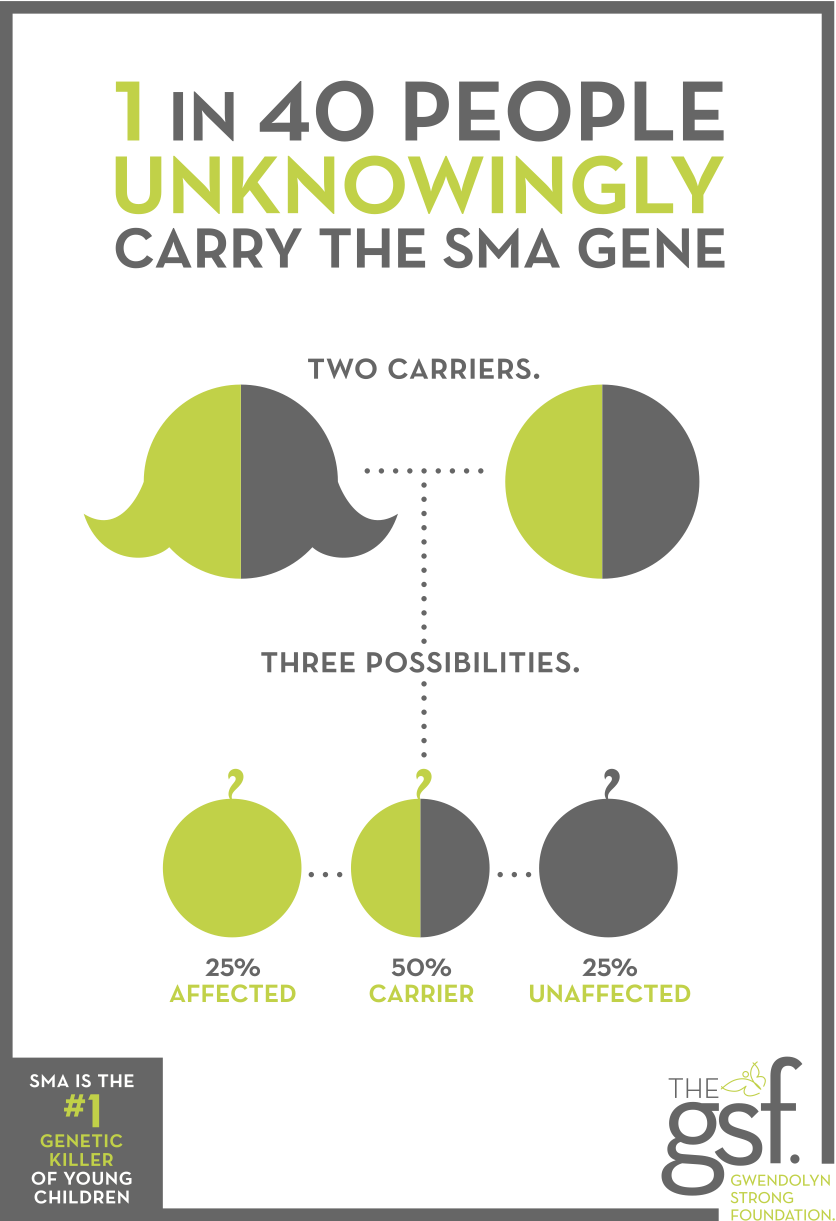
Increased carrier screening has always been an important issue to us as most parents do not learn they are SMA carriers until after their child is born with the disease — yet, 1 in every 40 unknowingly carry the SMA gene. Over the last nine years, we have advocated to increase public knowledge, created this infographic as a visual about carrier screening, have met with carrier screening companies and lent our story as an example of how carrier screening impacts lives.
We are so pleased that in their March 2017 publication, the American College of Obstetricians and Gynecologists (ACOG) published two Committee Opinions recognizing expanded carrier screening. Specifically and for the first time, spinal muscular atrophy (SMA) is now recommended to be offered to ALL women who are pregnant or planning to become pregnant, regardless of ethnicity or family history. (Previously only cystic fibrosis was recommended pan-ethnically.) The updated guidance also discusses the value of expanded carrier screening, which tests for up to several hundred conditions simultaneously.
In the finding, the Committee on Genetics stated: “Genetic conditions, including SMA, are not limited to one ethnic group. And certain conditions are common enough that it’s essential to offer screening for them in every patient.” Read the full Opinion here.
ACOG’s Opinions will have a national impact on changing medical policy and increasing access to carrier screening information as routine prenatal care. Their recommendation to offer testing to all couples counters the previously held approach in America. ACOG influences what is considered standard within OB practices, meaning because of this report more gynecologists around the country will now talk to their patients about expanded carrier screening that includes SMA — for many, BEFORE they are even pregnant! And now, with ACOG’s endorsement, more insurance companies will cover the cost of screenings for any couple interested.
So why didn’t ACOG recommend expanded carrier screening earlier?
Like many informed potential parents, I visited my OB before becoming pregnant and specifically asked what testing we should have done. When Gwendolyn was diagnosed we couldn’t understand why, when the technology was available, we weren’t given the opportunity to learn our SMA risk before pregnancy. Instead, Gwendolyn spent critical months with a misdiagnosis, delaying the use of life-extending interventions (like bipap, suction machine, feeding tube, and positioning) and increasing her risk of sudden death from malnourishment, choking, or breathing difficulties. It also meant we were completely blindsided and unprepared.
There were many facets to why the system was this way but much of it had to do with gene patents. Yes, the gene for SMA has a registered patent on it, which means the test for that gene falls under patent licensing. Thus, the company who owns and licenses the SMA gene determines the price of the test. In 2007, it was $1,000+ for one single gene test. A test that costs maybe $30 to run. A full panel of testing for the leading deadly genetic diseases would have been $100,000+ because companies own our genes, controlling knowledge about our own bodies. So, the lack of access to information had nothing to do with limited technology or ability to do these things. It came down to money and laws with unforeseen ramifications. Mad yet?
ACOG, as the leading authority on the standard practice of prenatal care, had to determine if it was ethically responsible for them to recommend expensive testing for a rare disease that the population may or may not be at increased risk. And, if you endorse SMA, what about other diseases? Where is the line drawn? So, for years, SMA carrier testing was only offered to families with a family history of the disease — sibling, cousin, etc., even though most newly diagnosed families have no history of the disease.
Enter startup companies that created commercially available expanded genetic testing panels inexpensively. (Like our friends and partners Counsyl.) These companies developed methods to collect blood or saliva that tests for several hundred conditions simultaneously at a fraction of the cost of single gene testing. Their technology and the belief that information about our individual genetic makeup belongs to each of us as individuals emboldened these companies to ignore the patents and even take on the very issue of gene patenting. And, with an ACLU lawsuit in 2009 against the idea of human gene patents on the basis that genes are not a man-made creation and, therefore, should not fall under patent law, the big gene patent owning companies stopped suing. With even more genetic screening companies now in the marketplace, the cost has become increasingly competitive and the number of diseases tested has become even bigger. This, in a very cliff noted version, gets us to ACOG’s new Opinion.
We are often asked why a couple would choose to have carrier testing. I always answer that carrier testing is very personal but the option of testing gives you the option for information. And knowledge is power. If a couple knows they are both carriers of a deadly genetic disease they may choose to pursue IVF with PGD, sperm donor, or embryo adoption. Or for the 7.5 million of couples who already require IVF, they can simply add PGD to the process. For other couples, knowing what’s in their genetic makeup may be a reason for them to choose adoption. And, for many other couples who learn they are carriers of SMA or another serious genetic disease, that knowledge allows them to go into a pregnancy knowing the risk so they can opt for a CVS or amnio during pregnancy to learn if their child has the disease. This is essential for preparation — emotional, financial, and now so you can get a treatment lined up.
Newborn screening is different from carrier screening, another important testing measure that has also been in discussion among government bodies, medical and genetic specialists, and advocacy groups for decades. Newborn screening is intended as a public health program to identify infants with treatable conditions before they present clinically and suffer irreversible damage.
Newborn screening is determined by individual states. However, most states follow the “Recommended Uniform Screening Panel (RUSP)” authored by the American College of Medical Genetics (ACMG) and commissioned by the Health Resources and Services Administration (HRSA). These governing bodies have held that new screenings are not added unless they meet certain criteria, one being that there is a treatment available. SMA experts, like Dr. Swoboda, have long been advocating that because early interventions in SMA (like bipap and a feeding tube) extend life, those methods should have been considered enough of a “treatment” to add SMA to the national newborn screening recommendation. There have also been pilot studies around the country to test accuracy and ease of SMA testing on newborns. But, it was never enough. Now, however, with the FDA approval of a more traditionally defined treatment, SMA meets the criteria. Advocacy efforts around the country are pushing individual states to consider adding SMA and, hopefully, very soon RUSP will be updated to include SMA.
Prenatal carrier screening does not replace newborn screening, nor does newborn screening replace the value of prenatal carrier screening.
Because the cost of the SMA treatment, Spinraza, is so astronomical at $750,000 in the first year, followed by an estimated $375,000 every year thereafter for the rest of a patient’s life, it obviously takes some time to get insurance approval and may necessitate finding new insurance. Studies have shown that the earlier the treatment is received, the greater the outcome. Meaning 1 day vs 1 month vs 3 months matters enormously on what abilities a child will have. At 3-month-old, a child with SMA Type 1 is already experiencing major muscle loss, impacting the ability to eat, breathe, hold the head up, etc. Time is critical. Prenatal knowledge allows families to more adequately prepare, meet with specialists, get insurance coverage ready, even select specific hospitals to deliver in so the baby could potentially have treatment immediately at birth in the same location while the mother is still recovering.
None of this is black and white. Nor is any of this a simple or singular issue. Bioethics will and should continue to be debated as technology will continue to expand our access to information. But, we will always believe that knowledge about our own bodies and the ability to make decisions about our health care are some of our most personal and fundamental rights. Knowledge is power. What one does with that knowledge is personal and will be varied. These decisions at the state and national level have major ramifications for decades. We are thrilled to see that SMA continues to be tackled from all directions and is consistently a trailblazer for other diseases.
Learn more about one expanded access test that includes SMA and 100+ serious genetic diseases from Counsyl.

